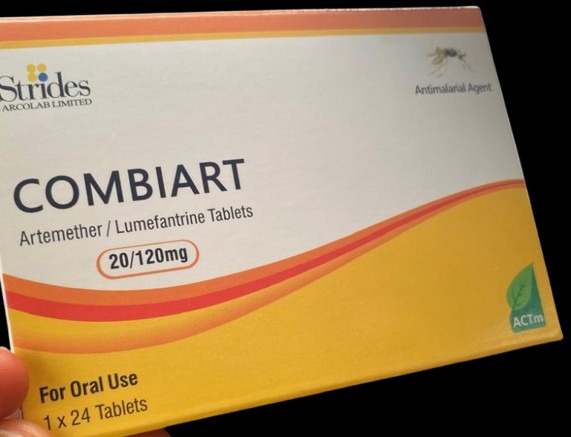
THE National Agency for Food and Drug Administration and Control (NAFDAC) issued a public health warning on Thursday evening regarding the circulation of a “counterfeit” malaria medication.
The drug, Combiart Dispersible Tablets (20/120 mg), “is manufactured by Strides Arcolab Limited, based in India. The product was discovered in FCT and Rivers State during surveillance activities,” NAFDAC said in a series of posts on X.
Strides Arcolab Limited has not reacted to the development.
The drug contains zero active ingredients, rendering it ineffective in treating malaria, NAFDAC added.
Malaria is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected mosquitoes, according to WHO.
The drug agency said the registration number on the drug packet was “incorrect” and that the drugs were manufactured between February and June 2023.
“Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office,” NAFDAC urged on X.
The agency stressed the drug lacks proper quality control, potentially leading to serious health consequences.
The drug also fails to effectively treat illnesses like malaria, allowing the disease to worsen, NAFDAC warned.
Trending
- Shettima: Nigeria ready to partner AU states on health security, sovereignty
- Fubara dedicates award to Wike for discovering him
- 2027 Election: Nigerians slam INEC for fixing polls in Ramadan
- INEC schedules dates for 2027 general elections
- National Assembly to harmonise electoral bill Monday as debate over electronic transmission intensifies
- African leaders shouldn’t be above age 50 —Jonathan
- Justice Egwuatu steps down from Malami’s trial over ‘personal reasons’
- Tinubu names Ismail Abba Yusuf new NAHCON chair, seeks Senate confirmation
- Appeal Court to hear suits challenging PDP’s Ibadan Convention Thursday
- Republican seeks visa ban on Kwankwaso, Miyetti Allah