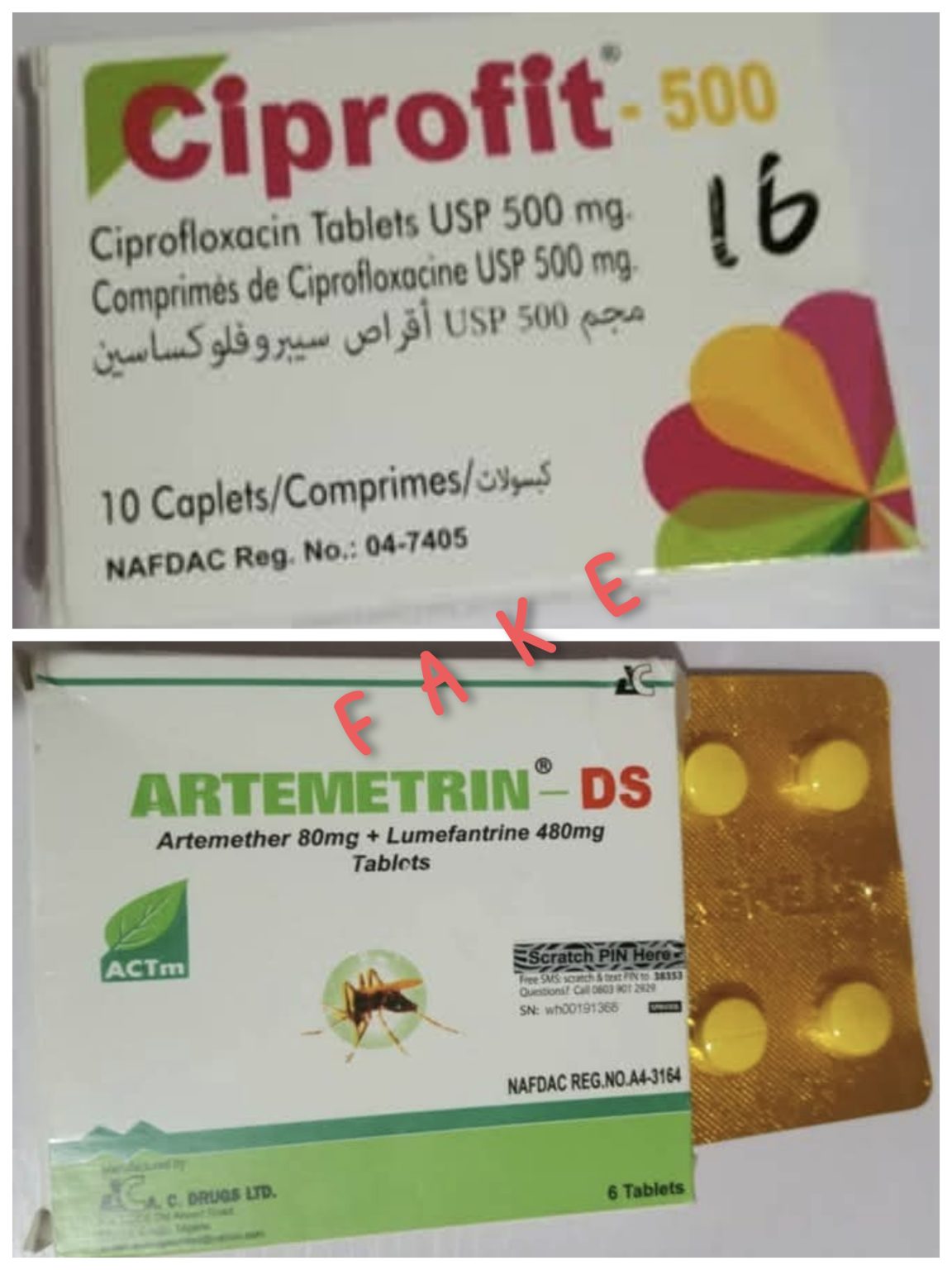
The National Agency for Food and Drug Administration and Control (NAFDAC) has alerted the public to the circulation of confirmed substandard and falsified medicines, specifically ARTEMETRIN DS (Artemether/Lumefantrine) and CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg), in Nigeria.
According to the agency, the ARTEMETRIN DS tablets, labelled as manufactured by A.C. DRUGS Ltd, Enugu, contained only 59.2% Artemether and 71.2% Lumefantrine, falling short of the acceptable 90-110% range.
Similarly, the CIPROFIT 500 tablets, said to be manufactured by Impact Pharmaceutical Ltd, Enugu, contained only 5.7% Ciprofloxacin, far below the required standard.
The findings followed an initial Thin-Layer Chromatography (TLC) test, which raised concerns about both products and led to further evaluation at a WHO-prequalified laboratory.
Read Also: NAFDAC DG warns on counterfeit products after football-linked arrest
NAFDAC noted that “the products do not exist on the NAFDAC registered products database, and all NAFDAC Registration Numbers stated on both products are false.” It further stressed that the medicines were purchased from a “licensed vendor and wholesaler,” raising concerns about counterfeit drugs penetrating regulated supply chains.
The agency urged members of the public to discontinue the use or sale of these products and return any stock to the nearest NAFDAC office. It added: “If you or someone you know has used any of these products and experienced adverse reactions, we strongly advise seeking immediate medical attention from a qualified healthcare professional.”
Healthcare providers and consumers have also been encouraged to report suspected cases of falsified medicines to NAFDAC through its offices nationwide, via the toll-free line 08001623322, or by email at [email protected]