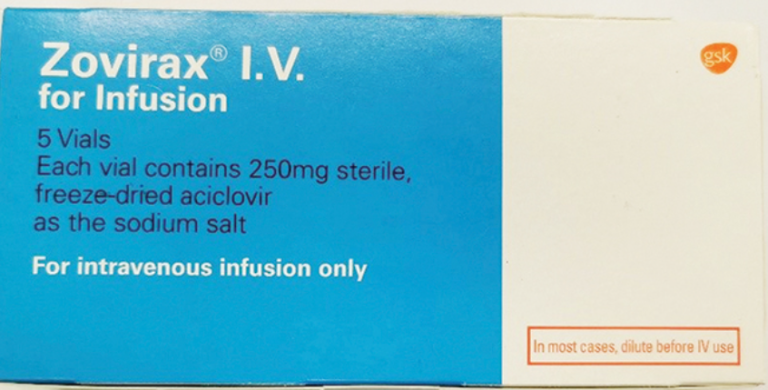
To prevent health complications and mortality, the National Agency for Food and Drug Administration and Control (NAFDAC) has issued a warning against the consumption of Zovirax IV Infusion 250mg RN3T following a recall in Hong Kong.
The manufacturer, GlaxoSmithKline (GSK) Limited, made the notification voluntarily after receiving a complaint from its branch in Taiwan that batch RP8K contained glass-like particles.
This was made via Nigeria’s anti-drug agency’s verified X account on Tuesday, stating that although the product was not listed in the NAFDAC database, it urged importers, distributors, retailers, healthcare providers, and patients to remain vigilant.
“As a precautionary measure, GSK is voluntarily recalling the affected batches of product from the market.”
“This is essential to prevent the importation, distribution, sale, administration, or use of recalled products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked,” NAFDAC noted.”
NAFDAC, however, following investigation, in its disclosure stated that the batch supplied to Hong Kong with batch number RN3T is considered to have the same potential quality issue as that of RP8K, as both batches were manufactured using the same batch of intermediates.
Zovirax IV Infusion 250mg, which contains Acyclovir as the main ingredient, is an antiviral agent used in the treatment of viral infections such as genital herpes, shingles, chickenpox, encephalitis, and other related conditions caused by the herpes zoster virus and varicella-zoster.
The drug is used to treat newborns who contracted Herpes simplex infections, immunocompromised patients, and severe initial genital herpes in non-immunocompromised patients.
According to NAFDAC, it highlighted that particulate matter contamination in infusions can lead to complications like phlebitis, thrombophlebitis, and granuloma formation, including pulmonary dysfunction and death.
The health agency urged medical professionals and the public to report any side effects, falsified or substandard medicine to the closest NAFDAC office, or dial 08001623322 or via email: [email protected].
“Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of the affected product to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med-safety application available for download on Android and iOS stores or via e-mail on [email protected].”